Affinity-based bioanalytical methods, such as immunoassay and DNA probing, rely largely on labeling technique. If the label is a small molecule, the signal-generating moiety is covalently linked to one or multiple functional group(s) of an analyte-specific biomolecule; while in certain competitive assays, the signal-generating moiety is coupled with a derivative or an analog of the analyte, which competes with the analyte in sample to bind to the analyte-specific biomolecule. In conventional ECL immunoassay, a bioconjugatable molecule, i.e., [Ru(bpy)3]2+ derivative such as [4-(N-succinimidyloxycarbonylpropyl)-4’-methyl-2,2’-bipyridine] bis-(2,2’-bipyridine)ruthenium (II) dihexafluorophosphate, is used as a label molecule. After the luminophore (signal-generating moiety) [Ru(bpy)3]2+ and the co-reactant tri-n-propylamine (TPA) undergo electrochemical oxidation and a cascade of follow-up reactions, a luminescent excited state, i.e., [Ru(bpy)3]2+*, is formed, and it emits detectable light.
There are many technical details in a real ECL immunoassay, which involves the way in which an antibody (in a sandwich assay) or an analyte (in a competitive assay) is labeled, how the anaylte is captured, how the ECL reactions are triggered and the working electrode is regenerated, etc. To take an ECL sandwich immunoassay as an example, in an typical commercial ECL assay, an antibody (signaling antibody) is labeled with a number of Ru(bpy)32+ units at the e-amino sites of the lysine residues and another antibody (capturing antibody) is biotinylated. When a clinical sample is mixed with the two types of antibodies and the streptavidin-coated magnetic microbeads for certain period of time, a sandwich immunocomplex is formed on the surface of the microbeads. The microbeads are then brought into an ECL measuring cell (a flow cell) and are captured on the surface of the working electrode by a movable magnet underneath. A TPA- containing buffer washes out the undesired substances and provides a chemical environment for Ru(bpy)32+ units to undergo the ECL reactions described below.
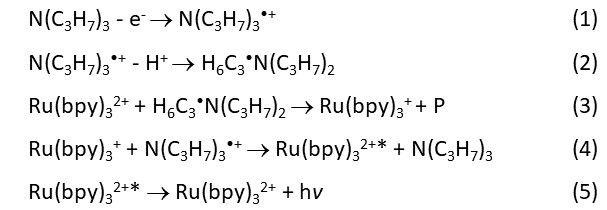
Other reaction schemes have been proposed to understand the formation of [Ru(bpy)3]2+* and the ECL generation (see J. K. Leland and M. J. Powell, J. Electrochem. Soc. 1990, 137, 3127-3131, and W. Miao, J.-P. Choi, A. J. Bard, J. Am. Chem. Soc. 2002, 124, 14478-14485). Some reactions occur theoretically only in homogeneous solutions, and cannot explain the ECL generated from the upper part of the micrometer-large magnetic beads.
Different from other chemiluminescence, [Ru(bpy)3]2+ undergoes the change only in the oxidation state, i.e.:
[Ru(bpy)3]2+ → [Ru(bpy)3]+ → [Ru(bpy)3]2+* → [Ru(bpy)3]2+,
without any chemical bond cleavage in the ECL process. Therefore, when TPA is in large excess, the ECL lasts a very long time until the applied potential is off. The integration of the number of photons over a certain period of time can be a measurement of the ECL intensity, often expressed as RLU (Relative Light Unit).
[Ru(bpy)3]2+* may undergo various reactions in different buffer systems. Some possible reactions are suggested in the figure at the top of the page.